With the end-point in mind: Focus on new pharmaceuticals
In April and May 2025, hundreds of representatives of the brightest R&D driven companies and academic and medical research teams gathered at Global Investors Forum, Innovation for Health and iDR25, with From Molecule to Business on 3 June 2025 still to come (attending is recommended!). Latest developments and trends were and will be discussed, and in the (panel) discussions current ideas and best practices were evaluated and challenged in view of improvements needed.
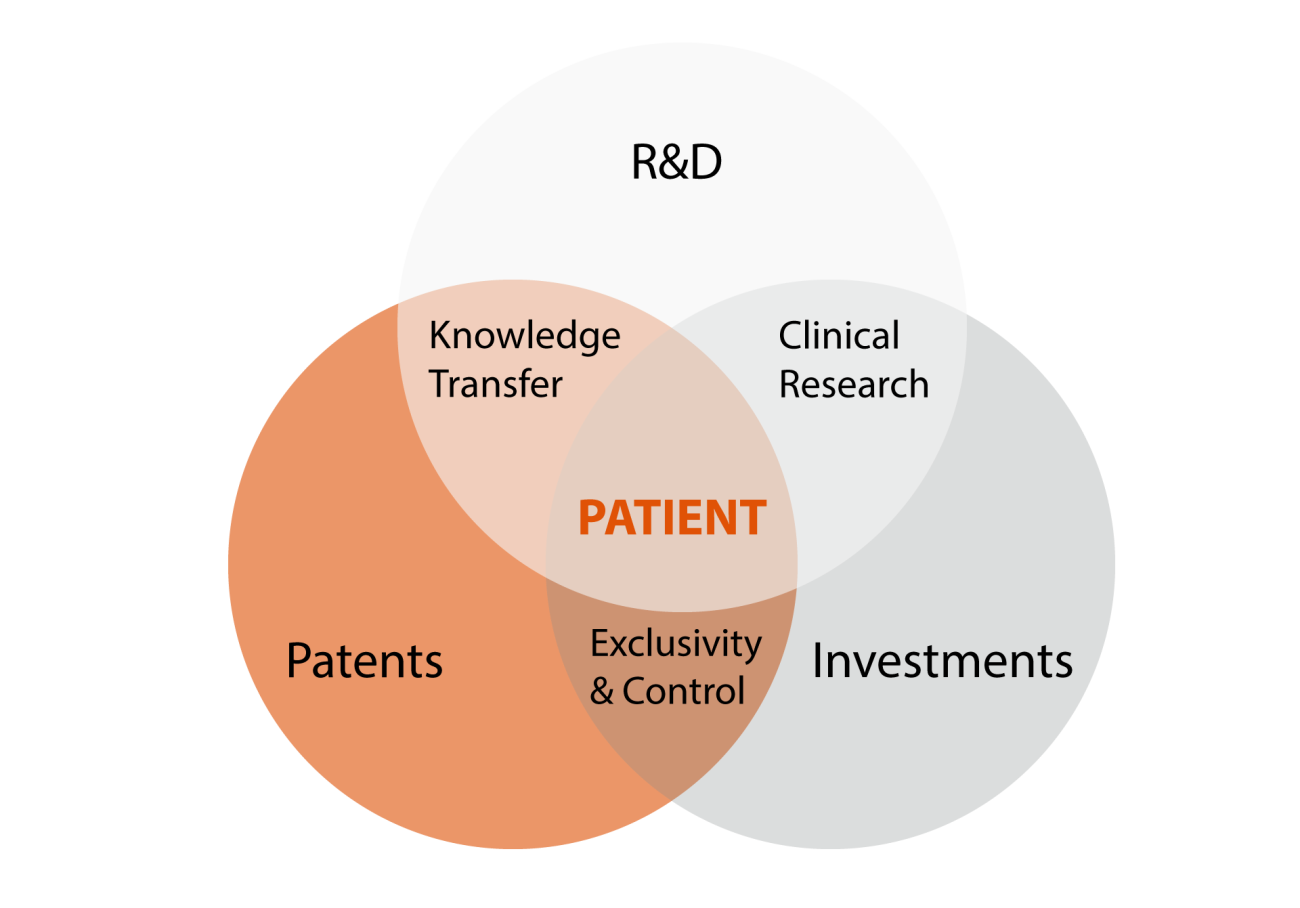
Identifying common themes complicating drug development progress
In the drug development field, many issues and discussions come down to four central themes:
- Access to sufficient financial resources at the right moment in time;
- The rather lengthy path from discovery till market authorization and ultimately a new pharmaceutical in the pharmacy and clinic (made accessible for patients);
- The recognition that experts on various different aspects of drug development are needed along the way to success and throughout the years, with the understanding that no single person combines all the knowledge and expertise in all fields: true team work required; and
- The uncertainty whether market authorization will be followed by reasonable and societal acceptable pricing, and in addition, by proper reimbursement schemes sufficient to start the effort and investment at all would such end-point be apparent right from the start, say 10-15 years ago.
The Dutch perspective: central role for academia in fuelling the drug development pipeline
Fortunately, in The Netherlands, it appears that there is still an enormous pool of innovative and ambitious individuals and teams willing and capable of starting and progressing drug development programs, despite the long and uncertain future ahead. Commonly, new ideas and initial promising data stems from academic research in universities, research centers and academic hospitals. The technology transfer offices (TTOs) play a decisive and pivotal role in valorisation of such potentially next generation drug development programs. New start-up and spin-off companies are established, internal and external qualified co-workers are teaming up and the journey starts.
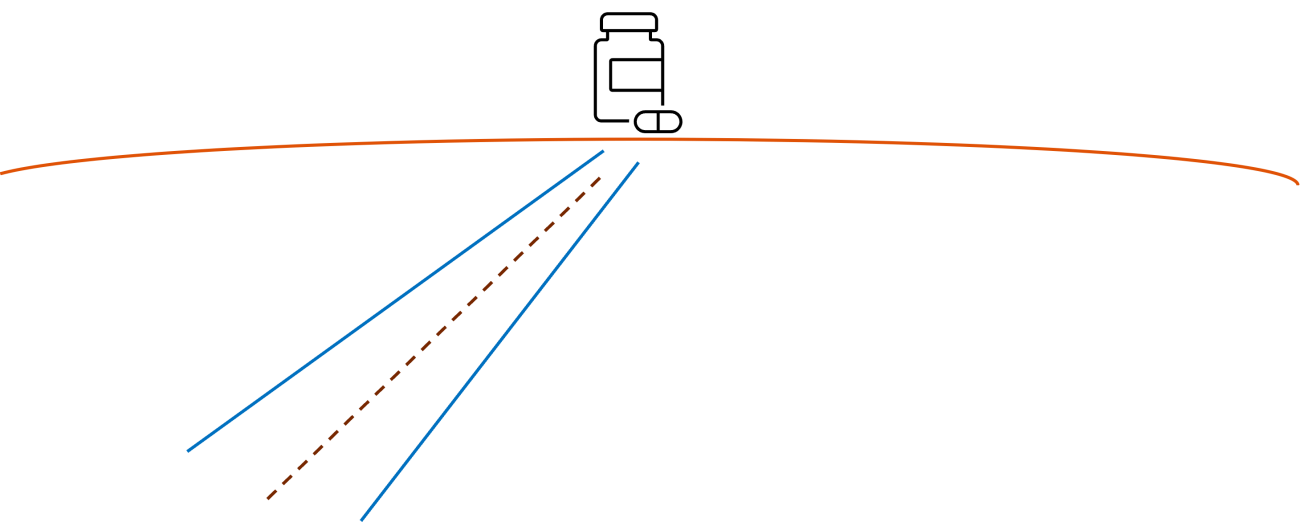
Life span of an early-filed patent and clinical trial data protection are not sufficient
It is virtually without exception that such research-driven initiation of a drug development program is accompanied by often a single or only a few patent applications filed by the TTO in close collaboration with the research group. Bearing in mind the quite regular time span till market authorization and distribution to pharmacies and hospitals of somewhere between 10 and 15 years, the life span of a patent, 20 years, with sometimes the option of expanding the protection to up to 25 years, might be too limited for allowing a proper return on investment, at first sufficient to cover costs and mitigate risks during the development program. In view of these timelines it is often heard that data protection for 10 years relating to conducted clinical trials for achieving market approval is filling in this gap. However, reality shows differently. When such data protection and patent protection relating to a patent filed in the early days of academic research lapse, further market protection is still essential for a viable and sustainable drug development program resulting in new therapies for patients.
Prolonged protection: rule of thumb for filing for longer patent protection, a practical approach
From filing a single patent application by a TTO, just before publication or thesis defence of the first glimpse on a potentially new therapy, to executing an established patenting strategy as an integral part of the drug development program; what is the gap apart from access to sufficient funding? It all starts with accepting that such single patent application filed in the early days will not be sufficient. Turning this insight into practice, filing new patent applications within 3-5 years from the first filing, within 5-8 and within 8-12 years, can for example be chosen as a healthy strategy and essential business aim. Easy said, but how? Some guidance is provided by the natural habit of those involved in a drug development program: if an inventor, R&D manager, CSO or CEO recognizes that new data is worthwhile presenting on a conference, network event, investors pitch or the like, and feels a strong need to take the stage, likely it is highly worthwhile at about 3 weeks in advance to inform the patent attorney that a new invention should be secured first, before the new pitch in the spotlights is delivered. The same for filing poster abstracts, publishing papers and submitting clinical trial protocols of which critical parts will be made publicly available. The rule of thumb is: if there is a need or felt urge to disclose new data in public, a new invention worthwhile patenting is most likely apparent. Overviewing timelines till for example clinical trials phase I, II and III, accompanying new insights and data become available at the indicated time suitable for prolonged patent protection beyond what data exclusivity would be able to provide. Thus nicely fitting with the designed patenting strategy.
Building a business in practice – team work acknowledging responsibilities and expertise
- Integrate a patent attorney in the core teams involved in drug development programs;
- Schedule periodic invention review and new invention identification meetings;
- Install a rather stringent gate keeping protocol, for clearing in time any and all publications stemming from the drug development program;
- Do not rely on non-disclosure agreements, only;
- Leave it up to the experts to assess which data is fit for valid and valuable patent protection at which moment in time;
- Understand the basics of patenting and appreciate that filing for a new patent application generally will take about 2-4 weeks of time, therewith in most occasions hardly interfering with timelines of other disciplines gathered in the drug development program.
Article as published in BiotechNEWS & Life Sciences on May 28, 2025.