Opportunities for the treatment of rare diseases with already available medicines
Repurposing medicines in the treatment of rare diseases
There are more than 7,000 known rare diseases, yet fewer than 10% have an available treatment. Fortunately, researchers in industry and academia are continually discovering new applications for existing medicines—a process known as drug repurposing.
For example, a drug originally developed to treat cardiovascular diseases was later found to be effective against benign tumours (haemangiomas) in children. This discovery enabled first-time access to treatment for affected patients. In other cases, repurposing improves existing therapies by enhancing effectiveness or safety.
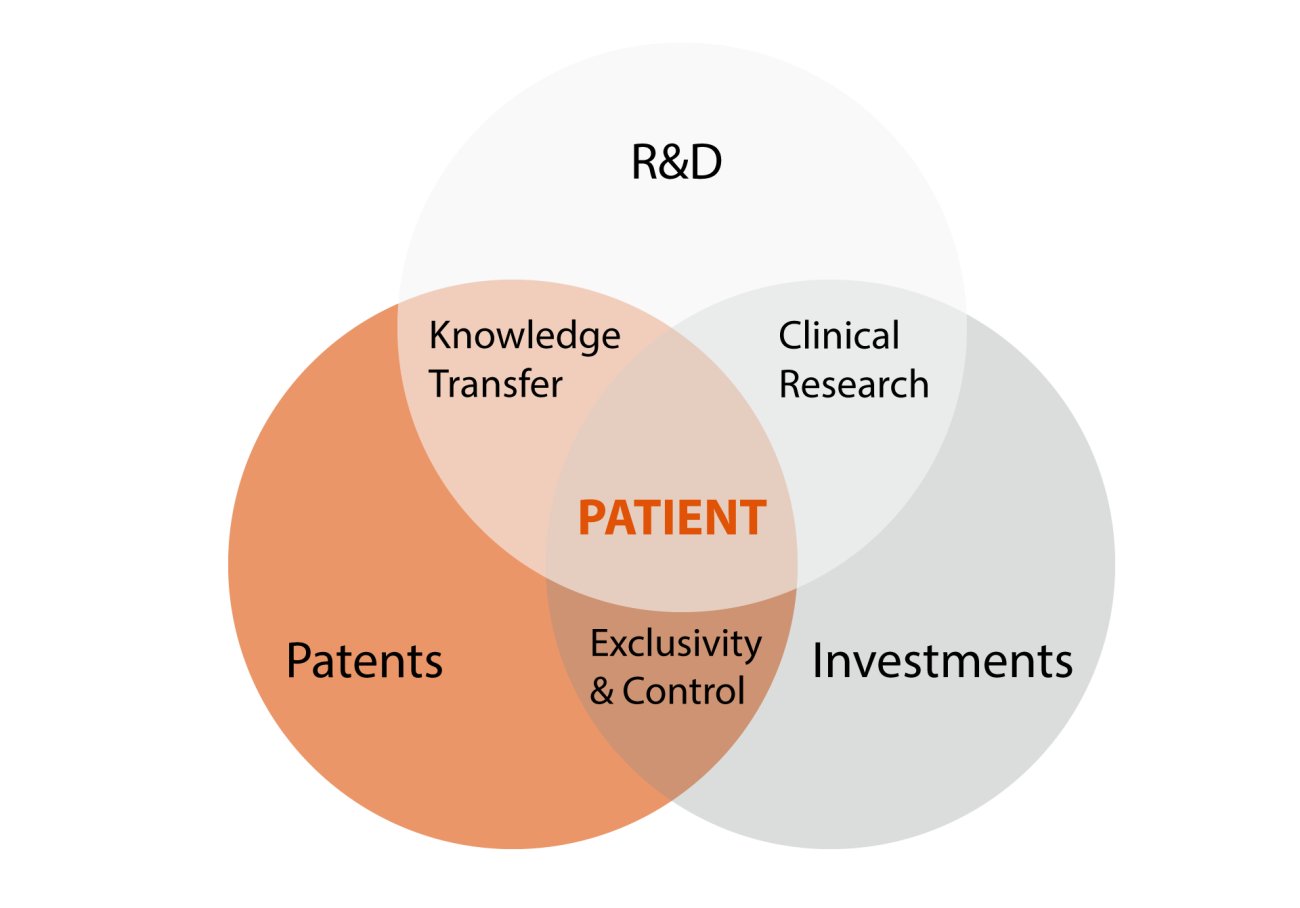
Additionally, repurposing can offer a faster and more cost-effective alternative to developing entirely new drugs. Patients benefit sooner, costs are lower, and access is improved. However, ensuring access to these treatments depends on the integration of high-quality clinical research, timely patent protection, and effective market regulation.
High investments: opportunities and risks
Developing a medicine—whether a new drug or an existing drug repurposed for a different disease—requires significant investment anywhere from several million to hundreds of millions of euros. This process involves:
- Extensive clinical research to ensure safety and efficacy
- Regulatory approval
- Production, logistics, marketing, and education before the therapy reaches patients
Since rare diseases often affect small patient populations, new treatments must typically be made available in multiple countries to ensure that the cost per treatment remains affordable.
Overcoming investment challenges in drug repurposing
Securing the necessary investment is often a major hurdle, especially when developing a repurposed drug. If the active ingredient in a medicine is still under patent protection, this can be an advantage—it allows innovators to safeguard their efforts and investments against competitors.
However, once a patent expires, the drug becomes a low-cost generic, making investment in repurposing more challenging. Even so, research into new uses for these medicines remains highly relevant for both academic and industry researchers, as well as for investors. The latter is because, in most cases, a new application for an existing drug can still be patented.
For example, a new patent could protect:
- A different rare disease treated with the same drug
- A new dosage form or method of administration for improving efficacy or ease of use
It is crucial that both pharmaceutical researchers and clinicians recognise these opportunities and act strategically and in a timely manner. Finding a new application for an existing drug in a new patient group is often the starting point for patent protection—providing comfort for both government and private sector investors.
Propranolol – a repurposed drug success story
A well-known example from literature is the drug propranolol, initially developed as an injectable treatment for high blood pressure. Later, researchers discovered its effectiveness for treating haemangiomas in children. This led to its introduction as an oral medication under a new patent.
This case highlights how a new formulation or method of administration for a rare disease can be patented, ensuring investment security and market exclusivity.
Discovering a new application for an existing drug in a new patient group is, almost by definition, the starting point for patent protection.
Strategy: securing control through patents
One of the key advantages of patenting a new application for a previously untreatable rare disease is the ability to control its development and commercialisation. However, securing investment for repurposing an existing drug can be particularly challenging, partly due to:
- The low pricing of generic medicines, which is often set by governments
- The limited reimbursement provided by health insurers
This makes recouping investment costs extremely difficult, especially considering that costs for clinical trials—even for existing drugs—can be rather high.
However, when the developers of the repurposed drug also hold the patent, they can effectively prevent competitors from entering the market. Unlike generic drug markets, where multiple companies compete over pricing and reimbursement, a patent grants exclusivity and full control over commercialisation.
To maximise success, it is essential to establish an exclusivity strategy from the earliest stages of drug development. This ensures that investors—whether public or private—have the potential to recoup their investments, given the long and costly process before market approval.
The role of tech transfer & collaboration
Academic hospitals often work with technology transfer offices (TTOs) or knowledge valorisation centres to manage patenting and commercialisation. These entities collaborate with patent attorneys to secure IP rights before seeking investors, as public funding alone is rarely sufficient to support the expensive clinical trials required for regulatory approval.
Collaboration across various stakeholders is essential, and several initiatives have been launched to support this effort. European consortia such as Repo4EU, Remedi4all, and the Dutch FAST programme focus on:
- Advancing drug repurposing
- Encouraging knowledge-sharing
- Facilitating cross-sector partnerships
In the Netherlands, ZonMW leads the ‘Good Use of Medicines’ programme, while at the European level, the EU Pharma Programme aims to harmonise healthcare regulations and encourage drug repurposing through policy frameworks.
By aligning patent protection, investment strategies, and collaborative efforts, repurposed drugs have a greater chance of reaching patients faster and more efficiently.
A Win-Win-Win for patients, innovators, and investors
Patent protection for a new therapy—whether it involves a new active ingredient, a new application for a new patient group, or a new method of administration the drug—offers significant benefits for patients. It ensures access to well-researched, safe treatments developed specifically for their needs. This could mean an improved therapy or even a completely new treatment for a previously untreatable rare disease.
For the inventor—whether a researcher, physician, or small pharmaceutical (start-up) company—securing patent protection for a new use of an existing drug achieves the ultimate goal: bringing new treatment options to patients. After all, this is what drives medical research and motivates everyone involved in the long and often difficult development process—including the patients themselves.
From an investment perspective, repurposed drugs can be particularly attractive, especially when protected by a patent. The risk is lower because the safety and health studies for the drug have often already been conducted. In the pharmaceutical industry, the majority of drug development projects fail before reaching the market, making investment in new drugs highly uncertain. However, for repurposed drugs, this risk is significantly reduced, as some of the costly clinical trials may no longer be required.
Faster and more cost-effective market entry
Patents play a crucial role in accelerating the market entry of repurposed drugs. A traditional drug development pathway can take 10 to 15 years, while bringing a repurposed drug to market can sometimes take as little as five years. This not only reduces costs but also expedites access to life-changing treatments for patients.
Patent protection is a key enabler in bringing repurposed drugs to market, ensuring that investment incentives align with patient needs. With the right strategies, collaborations, and policy support, drug repurposing has the potential to unlock life-changing treatments for many more rare diseases worldwide.
Patients are at the heart of drug development, with investment and patent protection playing a crucial role.