

A matter of life and death in Healthcare

Medical Devices Blogpost #1
“How can I best protect this specific medical implant I came up with?” In our patent practice we get lots of questions about how to protect medical technology, like surgical devices, implants, imaging devices and sensors. Also software and data analysis that is involved in converting collected signals and information into useful indications is inquired for frequently. Therefore my team and me will create a series of blogposts to inform you about this growing work field full of new developments and innovations. This our first post.
Avoid any risk in inventing medical devices
Medical devices are products that are intended to be used in diagnostics, treatment, and prevention in the healthcare sector. Because these products potentially can impose risks to the human well-being, medical products are generally governed by EU regulations. Risks are obvious for a surgical cutter or a stent, but also less obvious risks should be addressed. Take a blood glucose monitor as an example – you are never going to die from pricking your finger with the lance, but you might die if the algorithm designed to alert you to dangerously low glucose levels is flawed.
Stricter rules and regulations
New and upcoming regulations impose increasingly strict demands on medical devices, and on the manufacturers who produce or sell medical devices. Before you are permitted to market medical devices in several risk classes (*), you must first have them tested and approved by Notified Bodies. How can we not agree?

Innovations and improvements; regulations ensure top devices only
What if it was you, in need of a blood bag; you assume it’s sterile, but infections are lurking when these strict regulations would not be enforced. Also, it is a requirement to keep conducting active research into your products for as long as they are on the market, using stricter rules. Innovations and improvements; this regulation ensures only top devices on the market.
Examples of medical devices
- Instruments for performing surgery, e.g. stapler, cutters, ablation catheters
- Equipment for imaging the body, e.g. MRI machines, mapping catheters, X-ray apparatus
- Prosthetics and implants, e.g. a hip replacement, a prosthetic hand, breast implants
- Devices to be used on or in the eyes, e.g. contact lenses
- Non-pharmaceutical fillers, e.g. dermal fillers
- Home test devices, e.g. blood glucose meters, pregnancy tests, Covid-19 antigen self-test
- Delivery devices, e.g. syringes, intravenous drip bags
- Medical accessories, e.g. sutures, gloves, staples, fluid storage bags
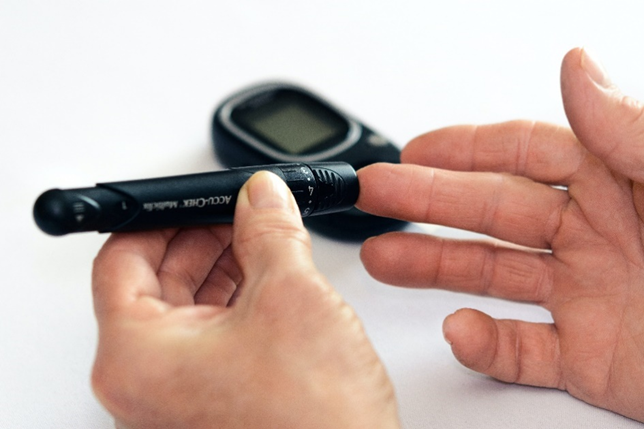
Securing and patenting medical devices is crucial
A dynamic market full of innovation is made possible by the continuous research and development efforts made by manufacturers and institutes. The protection of any resulting inventions is vital for safeguarding the return on investment, or even the survival of the company.
If you have any questions regarding IP protection of medical devices, do not hesitate to contact us via Healthcare | NLO.
For more information on protection of IP of any matter please subscribe to NLO’s LinkedIn account.
* In this article I refer to risk classes IIa, IIb, III and IVD B, C, D for medical devices. Companies are allowed to approve products from classes I and A (non-sterile and/or non-measuring) themselves.
Health Focus Group: NLO's special taskforce
You can read more articles on IP, healthcare and medical devices here:
- Methods of treatment by surgery: tread carefully | NLO
- Emerging technologies: what is trending? | NLO
For more information on protection of your intellectual property, please subscribe to NLO@LinkedIn or contact one of our IP experts: Healthcare | NLO.




